December 01, 2020
November 2020 brought a mixture of pandemic-related news. On the one hand, the world is experiencing the highest surges to date in number of cases and hospitalizations. But on the other hand, in recent weeks, we received very encouraging news. To date, three pharmaceutical companies have announced the results of their vaccine clinical trials, with demonstrated efficacy surpassing the most optimistic expectations. Countries around the world continue their scientific review to determine whether the vaccines will be approved for use, with UK being the first one to approve the Pfizer vaccine for use outside clinical trials. Emergency Use Authorization (EUA) process has commenced. As these important steps bring the world closer to translating this vaccine accomplishment into vaccination success, employers are wondering what role they can play in achieving that goal.
In order for COVID-19 to shift from pandemic to endemic, a critical mass of people must be vaccinated by any approved vaccine. For this to take place, a variety of factors must come together: approvals at the country level, equitable distribution, vaccine acceptance by the public, low cost, sufficient supply to meet demand, and accessibility. The demand for the vaccine is expected to grow, with public confidence increasing as safety data emerges. Therefore, to increase vaccine compliance, we all need to work to reduce barriers to access.
The virus has had an unprecedented impact on business and life in general. As a result, discussions about COVID-19 vaccination are set to dominate family and executive discussions alike for the foreseeable future. During this difficult period, the following key elements relevant to the future of COVID-19 vaccines are worth being aware of and considering:
Approval
Each country’s appropriate health authority, such as the Food and Drug Administration (FDA) in the U.S., will determine when each vaccine is approved for the country. This means that both the particular vaccines approved, as well as the timeline for approval, will likely vary by country.
Vaccine Types and Related Distribution Challenges
Messenger RNA (mRNA) technology-based vaccines are truly groundbreaking. This approach differs from traditional vaccination, where an inactive, non-infectious version of the virus is given to patients to instruct the immune systems to respond to the "antigens," or "spike proteins" on the outside of the virus. mRNA vaccines, on the other hand, provide the body with a specific messenger RNA molecule that has been engineered to express the same spike proteins; the patient's immune system responds the same way, but no actual virus is required.
According to Dr. Sanjay Gupta, CNN chief medical correspondent, the speed in which the vaccine was developed adds to the uniqueness of this scientific achievement. . It took exactly 248 days from idea to applying for EUA with the FDA for the Pfizer vaccine, a process that for other vaccines would be considered speedy if accomplished in 8 years.
Both the Pfizer/BioNTech vaccine and the Moderna vaccine are based on the mRNA technology. This new type of vaccine has two major advantages. First, because these vaccines are not made from the virus itself, the fear of being injected with the virus -- even the noninfectious form, present in traditional vaccines—is reduced. Second, the vaccines had no major side effects reported during phase 3 trials, pointing to their safety
Efficacy was also much higher than expected. Both manufacturers reported over 90% efficacy after two doses spaced either 21 or 28 days apart, much higher than the 50% efficacy floor set for approval by the FDA and the European Medicines Agency (EMA). As a result, the FDA is expected to grant the EUA quickly, and distribution of the Pfizer vaccine may begin in December.
The Pfizer vaccine requires the coldest temperatures for transport and storage; therefore, it is most likely to be distributed through pharmacies and large health care settings, which have ultra-cold storage facilities. Upon approval, Pfizer is expected to have 20 million doses available, with production ramping up quickly.
Moderna has stated that it is confident in its ability to produce 500 million doses per year after raising $1.3 billion through stock sale.2 These figures are important to consider as employers try to project when this vaccine may be available for the general public.
In quick succession to the initial set of vaccine good news, AstraZeneca and Oxford announced on November 23 their average phase 3 trial efficacy results of 70%. This rate is an average of participants in two studies, one in the U.K. which inoculated patients with half of the dose followed by the full dose a month later, (which achieved 90% efficacy), and a study in Brazil, where participants received two full doses a month apart. This study showed 62% efficacy. More research is required to understand the difference in the two approaches, as well as the difference in efficacy.
The AstraZeneca Oxford vaccine is more likely to benefit a wider population worldwide. At $3 to $4 per dose, it is also the least expensive of the three and should be easier to distribute because it can be stored in regular refrigerators. Lower-income countries around the world have been pre-purchasing this vaccine in preparation for distribution. As for capacity, AstraZeneca has said that it can supply 3 billion doses in 2021.
Two more pharmaceutical companies, Johnson & Johnson, which is developing a single-dose vaccine, and Novavax are expected to announce their results in the first quarter of 2021.
The EMA plays an important role in enabling the development, scientific evaluation, approval, and monitoring of COVID-19 vaccines in the European Union (EU). Vaccines for COVID-19 are being developed, evaluated and approved according to current regulatory guidelines and legal requirements. To gain approval for a vaccine in the EU, the vaccine developer submits the results of all testing investigations to the medicines regulatory authorities in Europe. This is part of a marketing authorization application.
Availability and Supply
Some employers have asked about sourcing vaccine supply directly and providing COVID-19 vaccination drives for employees, similar to flu vaccination drives. The supply and procurement related to a new vaccine for COVID-19, however, will be very different, as at least initially, demand for the vaccine will outweigh supply Therefore, sourcing will be handled at the country level. Some country governments have already begun preordering supply in anticipation of the vaccine’s approval. It is possible that some wealthier countries may be able to source and procure the vaccine before low-income countries, setting the stage for the potential for inequities in access. This is a significant risk that needs to be addressed proactively. Within countries, health equity could also be an issue if vulnerable populations are not prioritized. It is expected that in many locations, governments and national health systems will control supply to prioritize certain populations.
Safety and Distribution Priority
The difference between EUA and full approval is phase 4 results. Phase 4, also referred to as general surveillance, involves vaccinating the general population and collecting safety data on those vaccinated.
As the CDC is preparing vaccine distribution guidelines, vaccination safety initiatives are moving full speed ahead. In the U.S., the government, under the umbrella of the Vaccinate with Confidence framework, a strategic framework developed in 2019 to educate the public on vaccines and encourage their use, has designed new tools and adjusted existing tools for data tracking and information gathering. The Immunization Safety Office is responsible for vaccine safety monitoring and reports its findings and any concerns to CDC’s Advisory Committee on Immunization Practices (ACIP). This advisory group has been tasked with developing the COVID-19 vaccine prioritization framework. Based on the framework, the individual states will then develop their guidelines for distribution. This comes at the time when many states are already struggling with budget challenges as they fight the COVID-19 battle.
Many countries have already indicated that their governments and national health programs will control supply to prioritize certain populations. On September 16, 2020, the CDC released its first draft of guidance for COVID-19 vaccination distribution. The strategy includes “centralized distribution allowing the government full visibility, control, and the ability to shift assets and use data to optimize vaccine uptake.”3 With this model, employers will not be sourcing the vaccine directly, as the supply and distribution will be controlled centrally by the government and respective health agencies. Until a vaccine is authorized or approved by the FDA, specifics such as confirmation of the populations for whom the vaccine is most appropriate, dosage requirements and distribution particulars remain unknown.
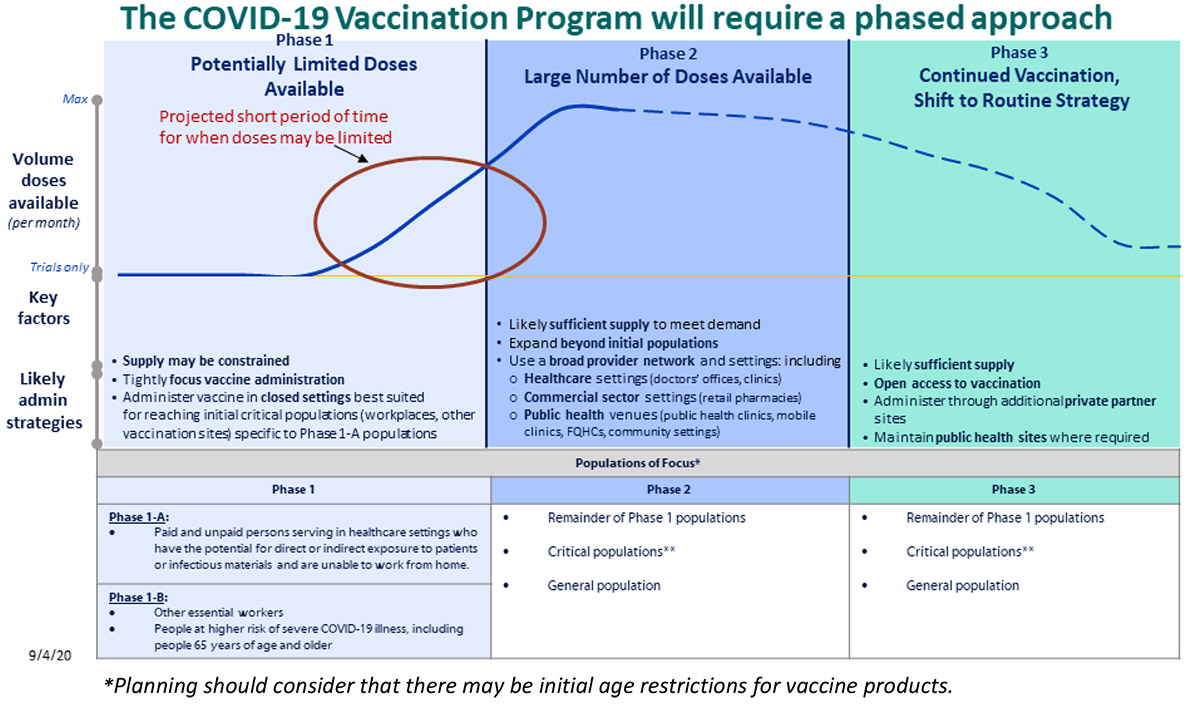
The U.S. plan outlines a three-phase structure (Figure 1) for distribution. The plan initially prioritizes certain populations in phase 1, when a limited number of doses will be available, expanding the eligible populations in phase 2, as availability of the vaccine increases. Phase 3 of this plan indicates that should the threat of COVID-19 persist as a public health need requiring ongoing vaccination, then COVID-19 vaccinations would eventually be integrated into routine vaccination programs for universal availability.
The U.K. has a similar phased approach for prioritizing populations according to age group. On December 8, the UK became the first country to begin vaccinating in accordance with their schedule. Phase 1, which is expected to take one month, includes older adult residents and workers in care homes, all those 80 years and older, and health care workers. Phase 2 is expected to take five months and includes ages 50 and above (with priority beginning with the oldest individuals first). The final phase 3, includes the remaining population, with further prioritization to be determined.
Affordability & Coverage
Another question raised by many employers has been related to the cost of the vaccine and if its coverage requirements will follow that of other vaccines in terms of reimbursement and out- of- pocket costs.
Many countries have indicated that the cost of the vaccine will be covered by their public health programs. In the U.S., the government will cover the cost of the vaccine for all Americans. The cost of vaccine administration, however, according to the provisions of the CARES Act, will be covered by the applicable health plan at no out- of- pocket cost, provided the vaccine is recommended by the U.S. Preventive Services Task Force or the CDC’s ACIP. Some employers are also considering covering members not insured through their plan via a vaccination program administered through a wellness benefit.
What Employers Can Do
Business Group on Health has heard from several member companies asking how they should be planning and preparing to support their employees around the world once a vaccine is available. At this point in time, there remain many unknowns about the vaccine distribution process and availability, making the ability for strategic preparation somewhat limited. Nonetheless, the role of the employers is becoming clearer, and there are some key steps that employers can take.
- Inform employees using scientific sources and data, which will also help to dispel myths. By acting as a source of evidence-based information, employers can play a critical role in health promotion and meeting the goal of having as many people as possible vaccinated as quickly as possible. Providing fact-based information about the effectiveness of vaccinations in general and sharing current information from health experts on the safety protocols and efficacy findings of any new vaccine can help build confidence and trust among employees.
- Monitor distribution guidelines and understand the role they can play in distribution. Initially, vaccine distribution will be controlled centrally to prioritize health care workers and high-risk vulnerable populations. By educating the workforce on who is eligible for vaccination, employers can increase the likelihood of their eligible employees receiving the vaccine. Employers in health industries and those with retail pharmacies or cold storage capabilities may be especially valuable collaborators with state governments.
- Advocating for priority populations: In the U.S., employers can work directly with state authorities responsible for defining the essential worker populations pursuant to the ACIP guidelines further advocating for their workforce, especially those who may have challenges accessing resources or vaccination sites.
- Assessing the pharmacy networks to ensure seamless distribution: According to the CDC, the U.S. government will purchase the COVID-19 vaccines from the manufacturers for controlled distribution, making the vaccine itself available to identified populations at no cost. Retail pharmacies are expected to play a crucial role in eventual broad vaccine distribution efforts. With that in mind, employers should work with their pharmacy benefit manager (PBM) partners to ensure that the networks are sufficiently broad to facilitate distribution. In addition, any contracting adjustments that govern pharmacy reimbursement for vaccine administration should be made ahead of time to ensure seamless access and coverage.
- Collaborating with Employee Resource Groups (ERGs): Involving ERGs, community leaders and employee advocates in communication campaigns can establish credibility and encourage those who would otherwise forego receiving the vaccine to do so. A recent U.S. study found that 52 % of Black respondents, and 34 % of Latino respondents said they would either definitely or probably not get the vaccine, despite the fact that the CDC states that Black and Latino individuals are 2.8 times more likely to die from COVID-19 compared to non-Hispanic white individuals.5 Such circumstances can deepen existing health inequities. Employers should seek to design any vaccine related communications in a culturally sensitive way.
- Understand and reduce barriers to access: To increase vaccination prevalence, we all need to work to reduce barriers to access. We all play a role in ensuring that the vaccine distribution process does not deepen existing health inequities. The pandemic has reminded us that infectious disease knows no boundaries and as a community, we are only as protected as our most disadvantaged citizens.
- Budget to cover any necessary costs of vaccine administration. In the U.S., collaboration with partners, especially PBMs, is key to setting up appropriate pharmacy networks and payment protocols for COVID-19 vaccine administration in pharmacy settings. For companies outside the U.S., once answers to the prerequisite questions related to approvals and distribution have become clearer, employers should work with their consultants and brokers to confirm if the COVID-19 vaccine(s) is covered by the public system. In addition, a company’s consultant and broker can help determine if discussions are needed with local carriers on whether coverage for the COVID-19 vaccine is included in their plan designs.
- Continuing to reinforce necessary workplace safety measures: While vaccine development is a critical step in changing the course of the pandemic, effective vaccination of millions of people is a complex and time-consuming task. Employers should continue to reinforce to their employee populations the importance of handwashing, social distancing and mask wearing at the same time as they share information about availability and accessibility of the vaccine.
- Revisit return-to-workplace plans based on vaccination distribution and prevalence. Consider how workplace strategy and worksite needs may be impacted by vaccine availability. In addition to vaccination rates, other factors, such as state positivity rates, masking, social distancing, and application of other public health measures. may allow workforces to return to the workplace before the majority of people receive the vaccine.
- Review sick and leave policies and decide if such policies need to be updated to include accommodations for employees needing time to access the vaccine or for those experiencing side effects.
- Facilitating vaccination in the future: In a CNN interview on November 10, Dr. Fauci said the timeframe for vaccine availability for the U.S. general population could be as early as April of 2021. With that timeframe a possibility, companies, especially those that have conducted flu vaccination drives, may consider their options for more active involvement in facilitating employee vaccination opportunities.
- 1 | Brian Walsh COVID-19 shows a bright future for vaccines.Axios. Nov 25, 2020 https://www.axios.com/vaccines-covid-19-coronavirus-future-f0b3f878-1c07-4bc4-b5e1-1169a76a1930.html. Accessed December 8, 2020
- 2 | Dan Stanton Moderna on $1.3bn manufacturing scale-up of mRNA COVID vaccine. BioProcess International August 6, 2020 https://bioprocessintl.com/bioprocess-insider/facilities-capacity/moderna-on-1-3bn-manufacturing-scale-up-of-mrna-covid-vaccine/ Accessed December 8, 2020
- 3 | Department of Health and Human Services. From the Factory to the Frontlines: The Operation Warp Speed Strategy for Distributing a COVID-19 Vaccine. https://www.hhs.gov/sites/default/files/strategy-for-distributing-covid-19-vaccine.pdf. Accessed September 21, 2020.
-
4 | Centers for Disease Control and Prevention COVID-19 Vaccination Program Interim Playbook for Jurisdiction Operations. October 29, 2020.
https://www.cdc.gov/vaccines/imz-managers/downloads/COVID-19-Vaccination-Program-Interim_Playbook.pdf Accessed December 8, 2020 - 5 | William Wan Coronavirus vaccines face trust gap in Black and Latino Communities, study finds. The Washington Post November 23, 2020 https://www.washingtonpost.com/subscribe/signin/?next_url=https%3A%2F%2Fwww.washingtonpost.com%2Fhealth%2F2020%2F11%2F23%2Fcovid-vaccine-hesitancy. Accessed December 8, 2020
More Topics
Articles & Guides
This content is for members only. Already a member?
Login
![]()